UPcare Selected & Invited by White House COVID Task Force to Discuss Collaboration
- Peter Xiao
- Nov 5, 2020
- 4 min read
Updated: Dec 3, 2024

UPcare Group focuses on developing a range of health care solutions that are all naturally based. They are scientifically tested and proven to be safe, effective and fast acting We pose significant commercial solutions for a number of areas in human health.
In response to the Covid-19 pandemic, UPcare Group has identified 3 natural compounds through it’s natural compounds discovery platform which has been operated jointly with Melbourne’s Monash University and a few other institutes.
UP-A, UP-U, and UP-P are 3 botanical extracts that have been selected by the Australian Anti-SARS-CoV-2 screening program as Priority 1 Group candidate. The compounds have shown potent anti SARS-CoV-2 virus activity in Vero cell and human lung cell infection experiments . UP-A has completed clinical phase 4 studies and consequently has proven activity in treating respiratory infections

Selected and invited by BARDA (Biomedical Advanced Research and Development Authority) of White House Coronavirus Task Force, on 5th Nov, Upcare scientists presented its 4 products (Upplus KBD Spray, Upplus KBD Chewable tablet, UPplus A KBD Spray and Upplus A capsule) developed from its anti SARS-CoV-2 virus compounds (UP-A, UP-P, and UP-U) to BARDA CoronaWatch meeting. Representatives from NIH, FDA, and CDC also joined the session and discussed COVID-19 solutions from UPcare.
During the BARDA CoronaWatch video meeting, UPcare scientists presented the discovery, testing, development and trials processes of the UPplus formulations, and how UPplus was selected as a candidate for the Anti-SARS-CoV-2 screening program, the panel showed strong interests in the exciting outcome of medical test findings. More importantly, all these compounds extracted from natural plants have been tested for safety with no side effect on human being. This discovery is a ground-breaking scientific breakthrough and it will be the most natural formulation available on the global market with almost zero side effects.

BARDA also invited UPcare to apply FDA Emergency Use Authorization (EUA) program once clinical trial finalised. UPcare Group hopes this meeting will streamline our EUA application process, which will enable us to fast track delivering both complementary and registered medicine to the people for prevention and treatment.
By 6 Nov, the U.S. reported more than 132,540 new coronavirus cases Friday by Worldmeters.info, the third day in a row the nation has recorded more than 100,000 new infections.


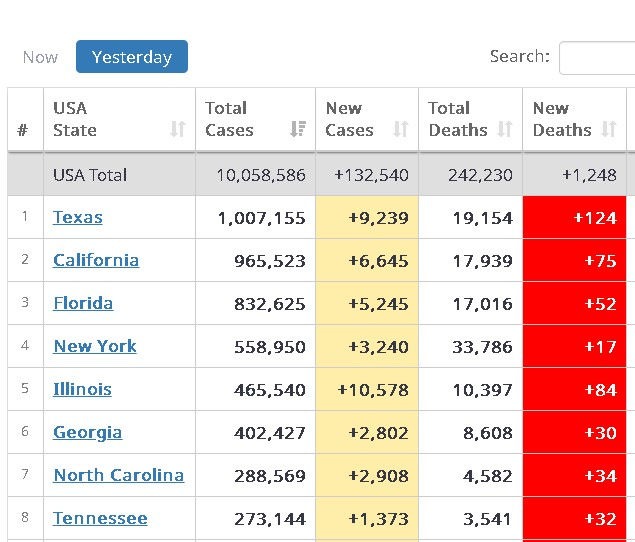
Infections are surging in all regions in the United States as the COVID-19 death toll continues to climb.

U.S. hospitalizations have significantly increased, forcing hospitals in Midwestern and Southern states to take urgent action to accommodate floods of new patients.
Midwestern states have been especially hard hit, with a record number of infections reported in Illinois, Indiana, Iowa, Minnesota, North Dakota, Nebraska and Oklahoma.


UPcare Group feels the pain for those people infected and their families, we have started to work with distributors and manufacturers in USA in order to deliver our products to USA market first to help contain, prevent and mitigate the spread.
About White House Coronavirus Task Force: The White House Coronavirus Task Force is a United States Department of State task force that "coordinates and oversees the administration's efforts to monitor, prevent, contain, and mitigate the spread" of coronavirus disease 2019 (COVID-19). Also referred to as the President's Coronavirus Task Force, it was established on January 29, 2020, with Secretary of Health and Human Services Alex Azar as chair. On February 26, 2020, U.S. vice president Mike Pence was named to chair the task force
About BARDA: Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services (HHS) office of the Assistant Secretary for Preparedness and Response, was established to aid in securing USA from chemical, biological, radiological, and nuclear (CBRN) threats, as well as from pandemic influenza (PI) and emerging infectious diseases (EID). BARDA supports the transition of medical countermeasures such as vaccines, drugs, and diagnostics from research through advanced development towards consideration for approval by the FDA and inclusion into the Strategic National Stockpile. BARDA’s support includes funding, technical assistance and core services, ranging from a clinical research organization network to Centers for Innovation in Advanced Development and Manufacturing, and a fill-finish manufacturing network.
About CoronaWatch: To enable a rapid-response to the COVID-19 outbreak, BARDA has repurposed its existing TechWatch program to focus on COVID-19 medical countermeasures. These CoronaWatch submissions and meetings aim to give innovators and innovative companies a government-wide platform to discuss their ideas with U.S. government experts, and seek partnership opportunities with a wide range of potential federal partners. BARDA is particularly interested in products and technologies that have progressed into or beyond non-clinical trials, have established large-scale cGMP manufacturing capability, or utilize an approved platform. Information regarding diagnostics, therapeutics, vaccines, and other products or technologies relevant to addressing this outbreak are sought.
ABOUT UPCARE: UPCare Group Pty Ltd was founded in Melbourne, Australia by a group of leading scientists, pharmacists and researchers to address the growing concerns and proliferation of viruses and other transmitted illnesses contracted through the human respiratory functions. The Group has collaborated with a number of biomedical research centers, and for many years has focused on research in three major areas: brain protection (reduced memory, senile dementia, and other diseases), lung protection (pharyngitis, rhinitis, pneumonia and other diseases) and diabetes.
About Monash University: Monash University was founded in 1958 and is the second oldest university in the state of Victoria in Australia. It is also the largest university in Australia. It was named after the engineer, military leader and public administrator Sir John Monash. It is a member of the Group of Eight, a group of Australia’s eight leading research universities.
the best university in Australia for Pharmacy and Pharmaceutical Sciences (Academic Ranking of World Universities, 2019)
2nd in the world for Pharmacy & Pharmacology (QS Subject rankings 2020)
7th in the world for Pharmacy and Pharmaceutical Sciences (Academic Ranking of World Universities, 2019)
14th in the world for Nursing (QS Subject rankings 2020)
30th in the world for Anatomy & Physiology (QS Subject rankings 2020)
equal 31st in the world for Medicine (QS Subject rankings 2020)
equal 39th in the world for Life Sciences & Medicine (QS Subject rankings 2020)
equal 46th in the world for Clinical, Pre-clinical Medicine and Health (Times Higher Education, 2017-2018)




Comments